A number of oxides can be used to produce acid rain.
Sulphur in coal will combine with oxygen to form sulphur dioxide.
Sulphur + oxygen  sulphur dioxide
sulphur dioxide
 sulphur dioxide
sulphur dioxide
S + O2  SO2
SO2
 SO2
SO2
Sulphur dioxide can react with water directly to form sulphurous acid
sulphur dioxide + water  sulphurous acid
sulphurous acid
 sulphurous acid
sulphurous acid
SO2 + H2O  H2SO3
H2SO3
 H2SO3
H2SO3
Sulphurous acid can react with oxygen and water to form sulphuric acid.
sulphur dioxide + oxygen + water  sulphuric acid
sulphuric acid
 sulphuric acid
sulphuric acid
2SO2 + O2 + H2O  2H2SO4
2H2SO4
 2H2SO4
2H2SO4
As stated in previous posts, vehicle exhausts produce nitrogen dioxide as follows
nitrogen + oxygen  nitrogen dioxide
nitrogen dioxide
 nitrogen dioxide
nitrogen dioxide
N2 + 2O2  2NO2
2NO2
 2NO2
2NO2
This in turn reacts with water to form nitric acid and nitrous acid
nitrogen dioxide + water  nitric acid + nitrous acid
nitric acid + nitrous acid
 nitric acid + nitrous acid
nitric acid + nitrous acid
2NO2 + H2O  HNO3 + HNO2
HNO3 + HNO2
 HNO3 + HNO2
HNO3 + HNO2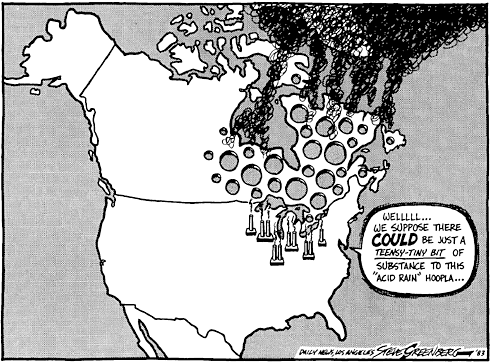
Acid rain forms in the atmosphere as these gases react with the water that is already in the atmosphere has a number of consequences. When it reaches the ground, it can have a number of consequences:
- Damaging the foliage of plants
- Rapid leaching of essential nutrients from the soils
- Releasing heavier metals from the soil (e.g. copper, zinc) so that they have toxic effects on plants.
- Acidifying waterways causing fish kills.
- Damaging statues or parts of buildings made of limestone and marble (some types of sandstone are also candidates for damage)
As the cartoon implies, the acid emissions of one country can affect neighbouring areas. This was the case in Canada where northern USA emission damaged the environment. In Scandinavia acid forming pollution from western Europe again caused damage.
In 1983 an international treaty, The Convention on the Long-Range Transport Air Pollutants (LRTAP) came into force with 50 signatories to try and reduce the emissions that produce acid rain. More on this later.
No comments:
Post a Comment